| Drug Name |
Cefuroxime axetil
|
| Synonyms |
cefuroxime axetil; Ceftin; Zinnat; Elobact; Zinat; Cefuroxime 1-acetoxyethyl ester; Bioracef; CXM-AX; Coliofossim; Furoxime; Cetoxil; Celocid; Cethixim; Nivador; Medoxm; Kalcef; Zoref; Sharox-500; UNII-Z49QDT0J8Z; DRG-0157; SN 407; CCI 15641; CCI-15641; Cepazine; CCI 1564; BRN 6854419; Z49QDT0J8Z; CHEBI:3516; (RS)-1-Hydroxyethyl (6R,7R)-7-(2-(2-furyl)glyoxylamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate, 7(sup 2)-(Z)-(O-methyloxime), 1-acetate 3-carbamate; C20H22N4O10S; Cefazine; Altacef
|
| Structure |
|
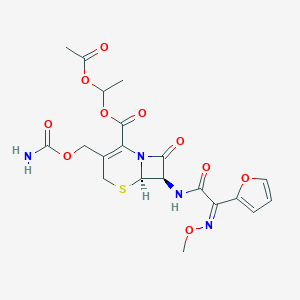 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 3 |
Molecular Weight (mw) |
510.5 |
|
| Logarithm of the Partition Coefficient (xlogp) |
0.9 |
| Rotatable Bond Count (rotbonds) |
12 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
12 |
| Chemical Identifiers |
- Formula
- C20H22N4O10S
- IUPAC Name
1-acetyloxyethyl (6R,7R)-3-(carbamoyloxymethyl)-7-[[(2Z)-2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate - Canonical SMILES
-
CC(OC(=O)C)OC(=O)C1=C(CSC2N1C(=O)C2NC(=O)C(=NOC)C3=CC=CO3)COC(=O)N
- InChI
-
InChI=1S/C20H22N4O10S/c1-9(25)33-10(2)34-19(28)15-11(7-32-20(21)29)8-35-18-14(17(27)24(15)18)22-16(26)13(23-30-3)12-5-4-6-31-12/h4-6,10,14,18H,7-8H2,1-3H3,(H2,21,29)(H,22,26)/b23-13-/t10?,14-,18-/m1/s1
- InChIKey
-
KEJCWVGMRLCZQQ-YJBYXUATSA-N
|
| Cross-matching ID |
- PubChem CID
- 6321416
- ChEBI ID
-
- CAS Number
-
- VARIDT ID
- DR01519
|
|
|
|
|
|
|
|

