Details of the Drug
General Information of Drug (ID: DM4NX0C)
| Drug Name |
Fevipiprant
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
872365-14-5; NVP-QAW039; QAW039; UNII-2PEX5N7DQ4; 2PEX5N7DQ4; 2-[2-methyl-1-[[4-methylsulfonyl-2-(trifluoromethyl)phenyl]methyl]pyrrolo[2,3-b]pyridin-3-yl]acetic acid; 2-(2-methyl-1-(4-(methylsulfonyl)-2-(trifluoromethyl)benzyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)acetic acid; 2-Methyl-1-[[4-(methylsulfonyl)-2-(trifluoromethyl)phenyl]methyl]-1H-pyrrolo[2,3-b]pyridine-3-acetic acid; Fevipiprant [INN]; Fevipiprant [USAN:INN]; QAW 039; Fevipiprant (JAN/USAN/INN); GTPL8995; SCHEMBL1940595; CHEMBL3137332; QAW-039; HMS3743E19; BCP25015; EX-A2495; BDBM50233520; ZINC43101772; AB85348; CS-5956; DB12011; Fevipiprant; NVP-QAW039; QAW039; SB16897; (1-(4-((Methane)sulfonyl)-2-trifluoromethylbenzyl)-2-methyl-1H-pyrrolo(2,3-b)pyridin-3-yl)acetic acid; 2-(1-((4-Methanesulfonyl-2-(trifluoromethyl)phenyl)methyl(-2-methyl-1H-pyrrolo(2,3-b)pyridin-3-yl)acetic acid; AC-31956; AS-74870; HY-16768; DS-022511; FT-0774596; D10631; Q27077292; 1-(4-methanesulfonylbenzyl)-2-methyl-1H-pyrrolo(2,3-b)pyridin-3-yl)acetic acid; (2-methyl-1-{[4-(methylsulfonyl)-2-(trifluoromethyl)phenyl]methyl}-1H-pyrrolo[2,3-b]pyridin-3-yl)acetic acid; [1-(4-Methanesulfonyl-2-trifluoromethyl-benzyl)-2-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl]-acetic acid; [2-METHYL-1-[4-(METHYLSULFONYL)-2-(TRIFLUOROMETHYL)BENZYL]-1H-PYRROLO[2,3-B]PYRIDIN-3-YL]ACETIC ACID; 2-(1-{[4-methanesulfonyl-2-(trifluoromethyl)phenyl]methyl}-2-methyl-1H-pyrrolo[2,3-b]pyridin-3-yl)acetic acid; 2-[2-methyl-1-[[4-methylsulfonyl-2-(trifluoromethyl)phenyl]methyl]pyrrolo[5,4-b]pyridin-3-yl]acetic acid; FSY
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
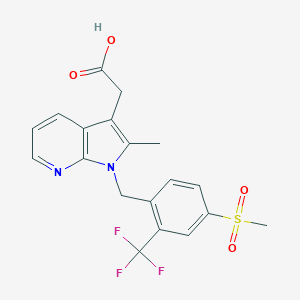 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 426.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 12 Disease of the respiratory system | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: CA23 Asthma | |||||||||||||||||||||||
| The Studied Tissue | Nasal and bronchial airway | |||||||||||||||||||||||
| The Studied Disease | Asthma [ICD-11:CA23] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
