Details of the Drug
General Information of Drug (ID: DM4OYXE)
| Drug Name |
LIAROZOLE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Liarozole; Liazal; 115575-11-6; R-75251; LIAROZOLE FUMARATE; Liarozole [INN:BAN]; Liarozolum [INN-Latin]; Liarozol [INN-Spanish]; R 75251; R-61405; R085246; R 61405; R-085246; R 085246; 6-((3-chlorophenyl)(1H-imidazol-1-yl)methyl)-1H-benzo[d]imidazole; UNII-17NYD2210B; CHEMBL389433; UNII-090Y06W08H; 17NYD2210B; 090Y06W08H; Liarozolum; Liarozol; 1H-Benzimidazole, 5-((3-chlorophenyl)-1H-imidazol-1-ylmethyl)-; NCGC00181034-01; Liarozole, (-)-; Liarozole, (+)-; AC1L1TNN; AC1Q3M3B; SCHEMBL18597; GTPL5210; SCHEMBL15944205; DTXSID9048277
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
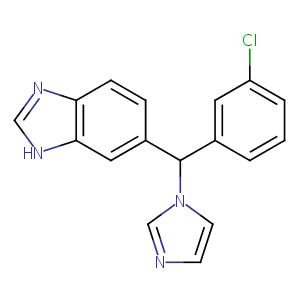 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 308.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
