Details of the Drug
General Information of Drug (ID: DM4YWCZ)
| Drug Name |
Nalmefene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alcofene; Arthene; Cervene; Cessal; Incystene; Nalmefeno; Nalmefenum; Nalmetrene; Nalmetreno; Nalmetrenum; Revex; Soberal; JF 1; ORF 11676; JF-1; NIH-10365; Nalmefeno [INN-Spanish]; Nalmefenum [INN-Latin]; Nalmetreno [INN-Spanish]; Nalmetrenum [INN-Latin]; ORF-11676; Revex (TN); Nalmefene (USAN/INN); Nalmefene [USAN:BAN:INN]; (5alpha)-17-(Cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinan-3,14-diol; (5alpha)-17-(Cyclopropylmethyl)-4,5-epoxy-6-methylenemorphinon-3,14-diol; (5alpha)-17-(cyclopropylmethyl)-6-methylidene-4,5-epoxymorphinan-3,14-diol; 17-(Cyclopropylmethyl)-4,5alpha-epoxy-6-methylenemorphinan-3,14-diol; 6-Desoxy-6-methylenenaltrexone; 9a-(Cyclopropylmethyl)-4,5alpha-epoxy-6-methylen-3,14-morphinandiol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticraving Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
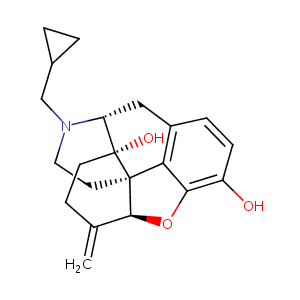 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 339.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
