Details of the Drug
General Information of Drug (ID: DM51FVE)
| Drug Name |
Zamifenacin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Zamifenacin; 127308-82-1; UNII-Y88Q418Y7M; Y88Q418Y7M; Zamifenacin [INN]; 127263-13-2; AC1MJ6I5; DSSTox_RID_82215; DSSTox_CID_27257; DSSTox_GSID_47257; SCHEMBL311829; CHEMBL27507; DTXSID9047257; UK-766542 [As Fumarate]; CHEBI:93417; ZINC3802222; BCP28100; Tox21_300210; AKOS022182209; SB19711; API0007427; UK-76654-2 [AS FUMARATE]; NCGC00247932-01; NCGC00254011-01; AJ-45652; CAS-127308-82-1; BRD-K80451230-051-01-8; (R)-3-(Diphenylmethoxy)-1-(3,4-(methylenedioxy)phenetyl)piperidene
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
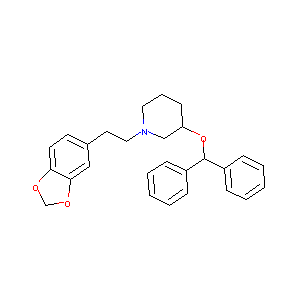 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 415.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Urinary incontinence | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | MF50.2 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
