Details of the Drug
General Information of Drug (ID: DM5GKOV)
| Drug Name |
Pergolide mesylate
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Pergolida; Pergolide (mesylate); Pergolide Methanesulfonate; Pergolide mesilate; Permax (TN); LY127809; PERGOLIDE MESYLATE; PERGOLIDE MESYLATE SALT; 55B9HQY616; 66104-23-2; 8-beta-((Methylthio)methyl)-6-propylergoline methanesulfonate; 8-beta-((Methylthio)methyl)-6-propylergoline monomethane sulfonate; 8beta-((Methylthio)methyl)-6-propylergoline monomethanesulfonate; CHEBI:8021; CHEMBL1275; CPD000058504; DSSTox_CID_20583; DSSTox_GSID_40583; DSSTox_RID_77029; MLS000069837; MPE; SMR000058504; UNII-55B9HQY616; LY-127,809; LY-127809; Pergolida [INN-Spanish]; Pergolide (INN); Pergolide [INN:BAN]; Pergolidum; Pergolidum [INN-Latin]; Permax; Prestwick0_000295; Prestwick1_000295; Prestwick2_000295; Prestwick3_000295; SR-01000721840; Spectrum2_001970; Spectrum4_000835; Spectrum5_001649; Spectrum_001647; TNP00315; pergolide; (8beta)-8-[(methylsulfanyl)methyl]-6-propylergoline; 24MJ822NZ9; 66104-22-1; CHEBI:63617; CHEMBL531; Ergoline, 8-((methylthio)methyl)-6-propyl-, (8beta)-; NCGC00017366-04; UNII-24MJ822NZ9
|
|||||
| Structure |
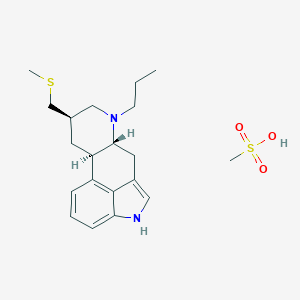 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 410.6 | ||||
| Logarithm of the Partition Coefficient | Not Available | |||||
| Rotatable Bond Count | 4 | |||||
| Hydrogen Bond Donor Count | 2 | |||||
| Hydrogen Bond Acceptor Count | 5 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
