Details of the Drug
General Information of Drug (ID: DM5JJ83)
| Drug Name |
Varespladib
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Varespladib; 172732-68-2; LY315920; Varespladib (LY315920); LY-315920; S-5920; VAREPLADIB; 2Q3P98DATH; ({3-[amino(oxo)acetyl]-1-benzyl-2-ethyl-1H-indol-4-yl}oxy)acetic acid; 2-(1-benzyl-2-ethyl-3-oxamoylindol-4-yl)oxyacetic acid; CHEMBL148674; S 5920; 2-((3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-indol-4-yl)oxy)acetic acid; 2-[[3-(2-Amino-2-oxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetic acid; 2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-indol-4-yloxy)acetic acid; SMR004701444; Varespladib [INN]; Varespladib [USAN]; Varespladib [USAN:INN]; UNII-2Q3P98DATH; varespladibum; ((3-(AMINO(OXO)ACETYL)-1-BENZYL-2-ETHYL-1H-INDOL-4-YL)OXY)ACETIC ACID; VRD; VARESPLADIB [MI]; Varespladib (USAN/INN); SCHEMBL26726; VARESPLADIB [WHO-DD]; MLS006010414; MLS006010980; varespladib methyl (A-002); varespladib sodium (A-001); LY 315920 (Varespladib); GTPL9657; DTXSID50169378; EX-A153; CHEBI:189668; BCPP000175; HMS3654E04; HMS3744O03; AMY19698; BCP01854; BDBM50055366; MFCD00944812; s1110; ((3-(aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl)oxy)acetate; AKOS015842901; compound 6m [PMID: 8978844]; BCP9000883; CCG-268404; DB11909; EX-8676; SB16690; NCGC00346491-01; NCGC00346491-02; NCGC00346491-08; NCGC00346491-12; AC-32931; AS-16956; HY-13402; A-001; FT-0659937; SW219559-1; A25228; D08107; EN300-144829; J-010846; Q7915613; BRD-K09711437-001-01-9; Z1721255146; (3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-acetic acid; 2-[2-ethyl-3-oxamoyl-1-(phenylmethyl)indol-4-yl]oxyethanoic acid; ((3-(Aminooxoacetyl)-1-benzyl-2-ethyl-1H-indol-4-yl)oxy)acetic acid; (3-Aminooxalyl-1-benzyl-2-ethyl-2,3-dihydro-1H-indol-4-yloxy)-acetic acid; 2-{[1-benzyl-3-(carbamoylcarbonyl)-2-ethyl-1H-indol-4-yl]oxy}acetic acid; ACETIC ACID, ((3-(AMINOOXOACETYL)-2-ETHYL-1-(PHENYLMETHYL)-1H-INDOL-4-YL)OXY)-; Acetic acid, ((3-(aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl)oxy)-,; LY 315920; Varespladib; 2-[[3-(2-Amino-2-oxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetic aci
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
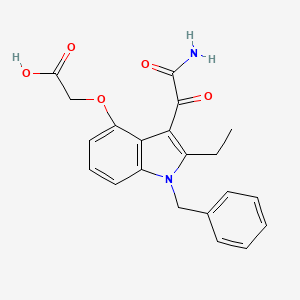 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Snakebite | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
