Details of the Drug
General Information of Drug (ID: DM67PWD)
| Drug Name |
Etilefrine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
etilefrine hydrochloride; 943-17-9; Etilefrine HCl; Circupon; Kertasin; Effontil; 534-87-2; 3-[2-(ethylamino)-1-hydroxyethyl]phenol hydrochloride; Effortilvet; Phetasin; Updormin; Pulsamin; Apocretin; Phetanol; Funasol; Ethyl adrianol; Tonus-Forte; Etilefrin Hydrochloride; Eti-Puren; 3-(2-(Ethylamino)-1-hydroxyethyl)phenol hydrochloride; 2-Ethylamino-1-(3-hydroxyphenyl)ethanol Hydrochloride; Etilefrine hydrochloride (TN); dl-Effortil hydrochloride; Ethylephrine hydrochloride; dl-Etilefrin hydrochloride; dl-N-Ethylnorphenylephrine h
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
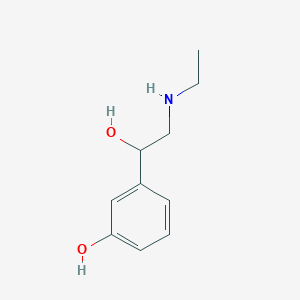 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 181.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
