Details of the Drug
General Information of Drug (ID: DM6V9SC)
| Drug Name |
ATL-844
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N-(4-Cyanophenyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)phenoxy]acetamide; MRS 1754; 264622-58-4; MRS-1754; MRS1754; CHEMBL273807; N-(4-cyanophenyl)-2-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)phenoxy]acetamide; N-(4-Cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)phenoxy]-acetamide; [3H]MRS1754; [3H]-MRS1754; NCGC00015689-01; Lopac-M-6316; Lopac0_000729; GTPL453; GTPL449; MLS002153324; SCHEMBL1222380; AC1O7G54; CTK8E7916; CHEBI:93269; AOB5542; DTXSID80424967; MolPort-023-276-567; HMS3373J21; HMS3268F13; HMS2230A06; ZINC4475274; BDBM50086170; AKOS027427086; AKOS024457276; EX-3306; ATL-618; ATL-GW-22; ATL-GW-8; A2B adenosine receptor antagonists, Adenosine/Ortho-McNeil; A2B adenosine receptor antagonists (oral, asthma/diabetes), Adenosine Therapeutics; A2B adenosine receptor antagonists (oral, asthma/diabetes), Clinical Data
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
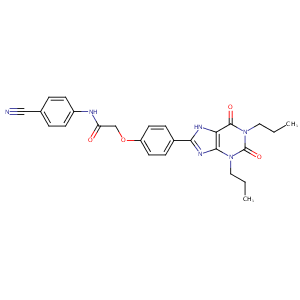 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 486.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
