Details of the Drug
General Information of Drug (ID: DM7EJ8S)
| Drug Name |
PF-06882961
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2230198-02-2; UNII-DN9IUI24GP; DN9IUI24GP; 2230198-02-2 (free acid); (S)-2-((4-(6-((4-cyano-2-fluorobenzyl)oxy)pyridin-2-yl)piperidin-1-yl)methyl)-1-(oxetan-2-ylmethyl)-1H-benzo[d]imidazole-6-carboxylic acid; 2-[(4-{6-[(4-cyano-2-fluorophenyl)methoxy]pyridin-2-yl}piperidin-1-yl)methyl]-1-{[(2S)-oxetan-2-yl]methyl}-1H-benzimidazole-6-carboxylic acid; Danuglipron; UK4; Danuglipron [USAN]; CHEMBL4518483; SCHEMBL20266351; BDBM349662; EX-A3607; WHO 11630; US10208019, Example 1A-09; US10208019, Example 4A-01; 2-[[4-[6-[(4-cyano-2-fluoro-phenyl)methoxy]-2-pyridyl]-1-piperidyl]methyl]-3-[[(2S)-oxetan-2-yl]methyl]benzimidazole-5-carboxylic acid; AC-31472; PF6882961; Q63141738; 1H-Benzimidazole-6-carboxylic acid, 2-((4-(6-((4-cyano-2-fluorophenyl)methoxy)-2-pyridinyl)-1-piperidinyl)methyl)-1-((2S)-2-oxetanylmethyl)-; 2-{[4-(6-{[(4-cyano-2-fluorophenyl)(methyl-d2)]oxy}pyridin-2-yl)piperidin-1-yl]methyl}-1-[(2S)-oxetan-2-ylmethyl]-1H-benzimidazole-6-carboxylic acid; C(#N)C1=CC(=C(COC2=CC=CC(=N2)C2CCN(CC2)CC2=NC3=C(N2C[C@H]2OCC2)C=C(C=C3)C(=O)O)C=C1)F
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
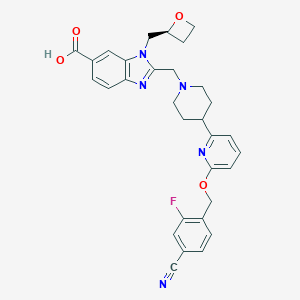 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 555.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 05 Endocrine, nutritional or metabolic disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 5A11 Type 2 diabetes mellitus | |||||||||||||||||||||||
| The Studied Tissue | Liver tissue | |||||||||||||||||||||||
| The Studied Disease | Type 2 diabetes [ICD-11:5A11] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
