Details of the Drug
General Information of Drug (ID: DM7JNTV)
| Drug Name |
Halaven
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Eribulin mesylate; Eribulin (mesylate); Eribulin mesilate; UNII-AV9U0660CW; Eribulin mesylate [USAN]; 441045-17-6; AV9U0660CW; CHEBI:70710; E 7389; E7389; Eribulin mesylate (USAN); B-1939; NSC-707389; Eribulin mesilate (JAN); CHEMBL1683544; QAMYWGZHLCQOOJ-WRNBYXCMSA-N; HY-13442A; AKOS030238218; CS-2803; D08914; 2-(3-amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9H,15H-furo(3,2-i)furo(2',3'-5,6)pyrano(4,3-b)(1,4)dioxacyclopent
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
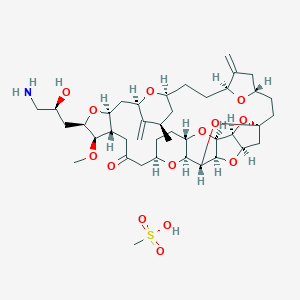 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 |
Molecular Weight | 826 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 15 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2010 | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Eribulin, a microtubule inhibitor for metastatic breast cancer. Oncology (Williston Park). 2011 Feb;25(2 Suppl Nurse Ed):46-8. | ||||
