Details of the Drug
General Information of Drug (ID: DM7PGOJ)
| Drug Name |
GSK2556286
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
GSK2556286; UNII-J9U8H9VQV0; GSK-286; J9U8H9VQV0; GSK-2556286; 1210456-20-4; GSK286; 6-((4-(2,3-Dimethylphenoxy)-1-piperidinyl)methyl)-2,4(1H,3H)-pyrimidinedione; 2,4(1H,3H)-Pyrimidinedione, 6-((4-(2,3-dimethylphenoxy)-1-piperidinyl)methyl)-; 6-[[4-(2,3-dimethylphenoxy)piperidin-1-yl]methyl]-1H-pyrimidine-2,4-dione; CHEMBL4650308; SCHEMBL19380190; EX-A6180; MS-24944; HY-147017; CS-0514179; Z644646478
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
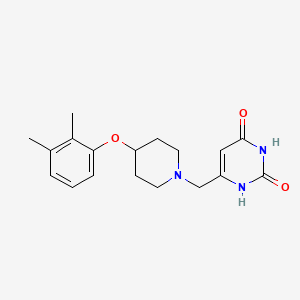 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
References
