Details of the Drug
General Information of Drug (ID: DM7U2BE)
| Drug Name |
Alloxazine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alloxazine; Isoalloxazine; 490-59-5; Alloxazin; benzo[g]pteridine-2,4(1H,3H)-dione; 1H-Benzo[g]pteridine-2,4-dione; Benzo(g)pteridine-2,4(1H,3H)-dione; UNII-880W3VF9YW; benzo[g]pteridine-2,4(3H,10H)-dione; CHEMBL68500; 880W3VF9YW; CHEBI:37325; HAUGRYOERYOXHX-UHFFFAOYSA-N; Benzo[g]pteridine-2,4[1H,3H]-dione; 1H,2H,3H,4H-benzo[g]pteridine-2,4-dione; Benzo[g]pteridine-2,3H)-dione; WLN: T C666 BN DMVMV INJ; 1,3-dihydrobenzo[g]pteridine-2,4-dione; SR-01000075199; Alloxazine, 96%; EINECS 207-714-3; NSC 203056; AC1NSYUA; Lopac-A-242
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
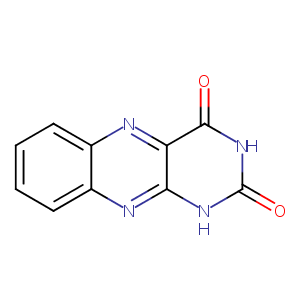 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 214.18 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
