Details of the Drug
General Information of Drug (ID: DM8IUV1)
| Drug Name |
ARV-766
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
ARV-766; ARV766; 2750830-09-0; SCHEMBL23536786; SCHEMBL23594097; GTPL12624; EX-A7714; NSC847604; NSC-847604; HY-153342; CS-0693933; 4-(4-((1-(4-((trans-3-(4-Cyano-3-methoxyphenoxy)-2,2,4,4-tetramethylcyclobutyl)carbamoyl)phenyl)piperidin-4-yl)methyl)piperazin-1-yl)-N-((S)-2,6-dioxopiperidin-3-yl)-2-fluorobenzamide; 4-[4-[[1-[4-[[[trans-3-(4-cyano-3-methoxyphenoxy)-2,2,4,4-tetramethylcyclobutyl]a mino]carbonyl]phenyl]-4-piperidinyl]methyl]-1-piperazinyl]-N-[(3S)-2,6-dioxo-3-piperidinyl]-2-fluoro-Benzamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
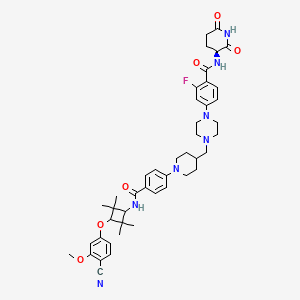 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Prostate cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C82.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
