Details of the Drug
General Information of Drug (ID: DM8QK2O)
| Drug Name |
5-benzyl-1,3,4-oxadiazole-2(3H)-thione
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5-benzyl-1,3,4-oxadiazole-2-thiol; 23288-90-6; 5-Benzyl-[1,3,4]oxadiazole-2-thiol; CHEMBL1164364; 5-Benzyl-1,3,4-oxadiazol-2-yl hydrosulfide; 1,3,4-Oxadiazole-2(3H)-thione,5-(phenylmethyl)-; 2-benzyl-1,3,4-oxadiazole-5-thiol; AC1Q7GGP; 5-benzyl-1,3,4-oxadiazole-2(3H)-thione; ChemDiv2_001277; AC1M00UR; SCHEMBL7023603; SCHEMBL11835354; CTK4F1247; DTXSID60365768; MolPort-000-473-148; HMS1649K04; HMS1372M01; ZINC2379869; BDBM50320724; STK498480; CCG-21122; BBL007772; AKOS000100218; AKOS001054851; MCULE-7500395690
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
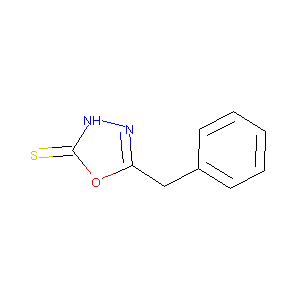 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 192.24 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
