Details of the Drug
General Information of Drug (ID: DM8TNX3)
| Drug Name |
Etizolam
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Etizolam [INN:JAN]; Etizolamum [INN-Latin]; VMZUTJCNQWMAGF-UHFFFAOYSA-N; Y 7131; Y-7131; etizolam; 4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine; 40054-69-1; 6-(o-Chlorophenyl)-8-ethyl-1-methyl-4H-s-triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine; 8-Ethyl-6-(o-chlorophenyl)-1-methyl-4H-s-triazolo(3,4c)thieno(2,3e)-1,4-diazepine; A76XI0HL37; AHR 3219; BRN 0572740; C17H15ClN4S; Depas; NCGC00182031-01; UNII-A76XI0HL37
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Structure |
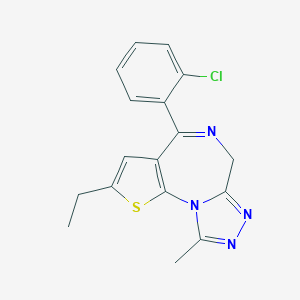 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 342.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
