Details of the Drug
General Information of Drug (ID: DM958QB)
| Drug Name |
Ripretinib
|
||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1442472-39-0; Ripretinib [USAN]; UNII-9XW757O13D; GTPL9175; SCHEMBL14999718; 9XW757O13D; HY-112306; CS-0044835; N-(4-bromo-5-(1-ethyl-7-(methylamino)-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2-fluorophenyl)-N'-phenylurea; 1-[4-bromo-5-[1-ethyl-7-(methylamino)-2-oxo-1,6-naphthyridin-3-yl]-2-fluorophenyl]-3-phenylurea; Urea, N-(4-bromo-5-(1-ethyl-1,2-dihydro-7-(methylamino)-2-oxo-1,6-naphthyridin-3-yl)-2-fluorophenyl)-N'-phenyl-
|
||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||||||
| Structure |
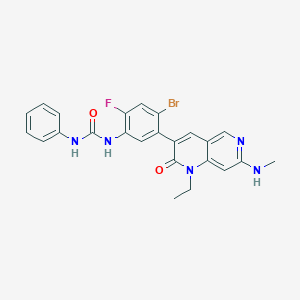 |
||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 510.4 | |||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.1 | ||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Gastrointestinal stromal tumour | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2B5B | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ripretinib (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020 | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | FDA Approved Drug Products: QINLOCK (ripretinib) tablets, for oral use | ||||
| 4 | Cerner Multum, Inc. "Australian Product Information.". | ||||
