Details of the Drug
General Information of Drug (ID: DM95Z4Q)
| Drug Name |
(R)-Rolipram
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(R)-(-)-Rolipram; 85416-75-7; (R)-ROLIPRAM; (4R)-4-[3-(CYCLOPENTYLOXY)-4-METHOXYPHENYL]PYRROLIDIN-2-ONE; R-Rolipram; UNII-DPX51KUP08; DPX51KUP08; CHEMBL430893; CHEBI:40133; 1xmy; NCGC00016899-01; CAS-61413-54-5; Tocris-1349; Tocris-0905; Tocris-1350; PubChem18284; 1ro6; 3g4k; 1q9m; AC1L9LJS; Lopac-R-6520; SCHEMBL576805; ZINC4982; KS-00000QDB; MolPort-003-983-801; HMS3267P19; BCP14111
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
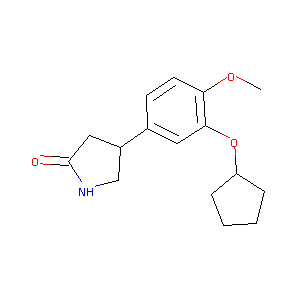 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 275.34 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
