Details of the Drug
General Information of Drug (ID: DM97I5L)
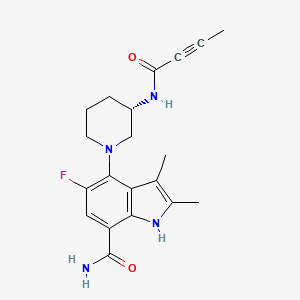
| Drug Name |
Branebrutinib
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Branebrutinib; BMS-986195; 1912445-55-6; (S)-4-(3-(but-2-ynamido)piperidin-1-yl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 7LBRZUYSHU; Branebrutinib [USAN]; BMS986195; 4-[(3S)-3-(but-2-ynoylamino)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; Branebrutinib (USAN); 1H-Indole-7-carboxamide, 5-fluoro-2,3-dimethyl-4-((3S)-3-((1-oxo-2-butyn-1-yl)amino)-1-piperidinyl)-; 4-((3S)-3-(But-2-ynamido)piperidin-1-yl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 4-[(3S)-3-(but-2-ynamido)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; UNII-7LBRZUYSHU; BRANEBRUTINIB [INN]; BRANEBRUTINIB [WHO-DD]; GTPL9869; CHEMBL4297674; SCHEMBL17699728; Branebrutinib (BMS-986195); C20H23FN4O2; VJPPLCNBDLZIFG-ZDUSSCGKSA-N; BDBM164638; BDBM166759; BCP29496; EX-A2720; MFCD31631584; NSC807627; s8832; WHO 11026; AKOS037649047; DB15347; NSC-807627; AC-31535; BS-16393; BMS986195; BMS986195; HY-112161; CS-0043577; Example 223 [US20160115126A1]; D11478; EN300-2007801; US9688629, 123; US9688629, 223; Q50825082; 4-((3S)-3-(2-Butynoylamino)-1-piperidinyl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; (S)-4-(3-(2-BUTYNOYLAMINO)PIPERIDIN-1-YL)-5-FLUORO-2,3-DIMETHYL-1H-INDOLE-7-CARBOXAMIDE; 4-((3S)-3-(2-BUTYNOYLAMINO)-1-PIPERIDINYL)-5-FLUORO-2,3-DIMETHYL-1HINDOLE-7-CARBOXAMIDE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 15 Disease of the musculoskeletal system/connective tissue | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: FA20 Rheumatoid arthritis | |||||||||||||||||||||||
| The Studied Tissue | Synovial tissue | |||||||||||||||||||||||
| The Studied Disease | Rheumatoid arthritis [ICD-11:FA20] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
