| Drug Name |
Methyl 2-amino-4-phenylthiophene-3-carboxylate
|
| Synonyms |
methyl 2-amino-4-phenylthiophene-3-carboxylate; 67171-55-5; 2-Amino-4-phenyl-thiophene-3-carboxylic acid methyl ester; 2-Amino-4-phenylthiophene-3-carboxylic acid methyl ester; 2-amino-4-phenylthiophene-3-carboxylic acidmethyl ester; AC1LEGNM; SMR000122717; ChemDiv3_014276; MLS000523644; SCHEMBL688523; METHYL2-AMINO-4-PHENYLTHIOPHENE-3-CARBOXYLATE; CHEMBL1456242; CTK6I8713; ZINC52966; DTXSID30350681; HMS1513I20; BDBM139491; HMS2354F24; KS-00003KD2; ALBB-001671; BBL015795; SBB006989; MFCD01050444; 7878AE; STK256668; AKOS000220131; FS
|
| Drug Type |
Small molecular drug
|
| Structure |
|
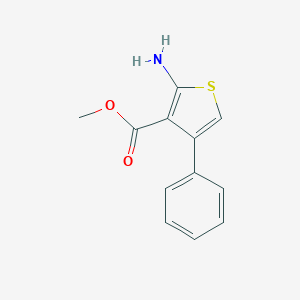 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
233.29 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.3 |
| Rotatable Bond Count (rotbonds) |
3 |
| Hydrogen Bond Donor Count (hbonddonor) |
1 |
| Hydrogen Bond Acceptor Count (hbondacc) |
4 |
| Chemical Identifiers |
- Formula
- C12H11NO2S
- IUPAC Name
methyl 2-amino-4-phenylthiophene-3-carboxylate - Canonical SMILES
-
COC(=O)C1=C(SC=C1C2=CC=CC=C2)N
- InChI
-
InChI=1S/C12H11NO2S/c1-15-12(14)10-9(7-16-11(10)13)8-5-3-2-4-6-8/h2-7H,13H2,1H3
- InChIKey
-
KHNSKPUYBBZGLW-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 685886
- CAS Number
-
- TTD ID
- D03VKC
|
|
|
|
|
|
|
|


