Details of the Drug
General Information of Drug (ID: DMA4BRV)
| Drug Name |
Elinogrel
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Elinogrel; 936500-94-6; UNII-915Y8E749J; 915Y8E749J; 5-CHLORO-N-[[[4-[6-FLUORO-1,4-DIHYDRO-7-(METHYLAMINO)-2,4-DIOXO-3(2H)-QUINAZOLINYL]PHENYL]AMINO]CARBONYL]-2-THIOPHENESULFONAMIDE; PRT-060128; Elinogrel [USAN:INN]; PRT 060128; Elinogrel (USAN/INN); SCHEMBL160663; CHEMBL2103828; MolPort-035-941-202; ZINC43153259; BDBM50397204; AKOS025142086; NCGC00387478-01; AN-26210; FT-0724857; D09607; 500C946; 1-(5-chlorothiophen-2-yl)sulfonyl-3-[4-[6-fluoro-7-(methylamino)-2,4-dioxo-1H-quinazolin-3-yl]phenyl]urea
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
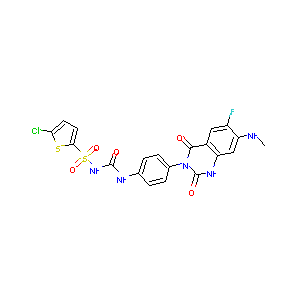 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 523.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
