Details of the Drug
General Information of Drug (ID: DMACPQO)
| Drug Name |
Dihydrexidine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
DIHYDREXIDINE; (+)-Dihydrexidine; UNII-Q3PJ4B4D0X; Q3PJ4B4D0X; CHEMBL25856; Dihydrexidine hydrochloride; (6aR,12bS)-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine-10,11-diol; (6aR,12bS)-5,6,6a,7,8,12b-Hexahydrobenzo(a)phenanthridine-10,11-diol; Rel-Dihydrexidine; Dihydrexidine, (+)-; Lopac-D-5814; Biomol-NT_000033; AC1O7G2G; Lopac0_000380; 5,6,6a,7,8,12b-Hexahydro-benzo[a]phenanthridine-10,11-diol; BPBio1_001227; SCHEMBL4614575; CHEBI:124993; DAR-0100; IP-202; BDBM50010686; ZINC25758512; CCG-204474; NCGC00024845-04
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
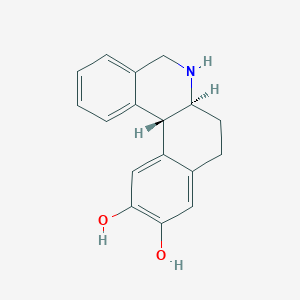 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 267.32 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Psychotic disorder | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20-6A25 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
