| Synonyms |
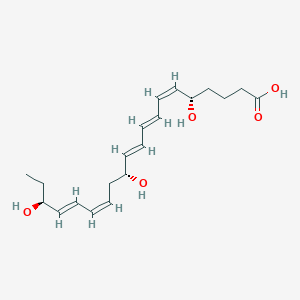
Resolvin E1; RvE1; 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid; UNII-GND3JH08JA; GND3JH08JA; 552830-51-0; resolvinE1; (5S,6Z,8E,10E,12R,14Z,16E,18R)-5,12,18-trihydroxyicosa-6,8,10,14,16-pentaenoic acid; 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-EPA; FA(20:5(OH3)); GTPL3333; SCHEMBL3321593; CHEBI:81559; ZINC56875015; LMFA03140003; DB13105; J2218407E; C18171; (5S,12R,18R)-trihydroxy-(6Z,8E,10E,14Z,16E)-eicosapentaenoic acid; (5S,12R,18R)-trihydroxy-(6Z,8E,10E,14Z,16E)-icosapentaenoic acid; [3H]RvE1; [(3)H]-labeled RvE1; resolvin E1
|
| Chemical Identifiers |
- Formula
- C20H30O5
- IUPAC Name
(5S,6Z,8E,10E,12R,14Z,16E,18S)-5,12,18-trihydroxyicosa-6,8,10,14,16-pentaenoic acid - Canonical SMILES
-
CC[C@@H](/C=C/C=C\\C[C@H](/C=C/C=C/C=C\\[C@H](CCCC(=O)O)O)O)O
- InChI
-
InChI=1S/C20H30O5/c1-2-17(21)11-8-5-9-14-18(22)12-6-3-4-7-13-19(23)15-10-16-20(24)25/h3-9,11-13,17-19,21-23H,2,10,14-16H2,1H3,(H,24,25)/b4-3+,9-5-,11-8+,12-6+,13-7-/t17-,18-,19+/m0/s1
- InChIKey
-
AOPOCGPBAIARAV-JJVMZPRHSA-N
|