Details of the Drug
General Information of Drug (ID: DMAXBOU)
| Drug Name |
6-Methyl-2-phenyl-chromen-4-one
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
6-Methylflavone; 29976-75-8; 6-methyl-2-phenyl-4H-chromen-4-one; 6-methyl-2-phenylchromen-4-one; ST069348; 4H-1-Benzopyran-4-one,6-methyl-2-phenyl-; AC1Q6AKA; AC1LEN9R; Maybridge3_003437; Cambridge id 5479507; Oprea1_512723; BIDD:ER0442; SCHEMBL4648879; CHEMBL134291; ZINC58123; CTK4G4151; DTXSID40351008; MolPort-000-648-469; NOQJBXPAMJLUSS-UHFFFAOYSA-N; HMS1440M05; HY-N6630; KS-000017TV; SBB012439; 6019AH; AKOS002387497; MCULE-3565329436; CCG-250475; IDI1_014824; NCGC00142612-01; ZB002324; 6-Methylflavone solution, 20 mM in DMSO
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
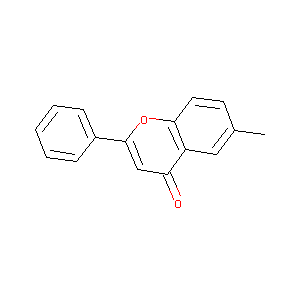 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 236.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
