Details of the Drug
General Information of Drug (ID: DMAZHT0)
| Drug Name |
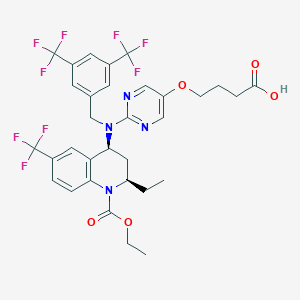
TA-8995
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Obicetrapib; UNII-8O74K609HN; 8O74K609HN; 866399-87-3; Obicetrapib [INN]; CHEMBL3785197; SCHEMBL17002081; AMG-899; DEZ-001; Obicetrapib (AMG-899 TA-8995); AKOS032954130; HY-18778; 4-((2-(((3,5-Bis(trifluoromethyl)phenyl)methyl)((2R,4S)-1- (ethoxycarbonyl)-2-ethyl-6-(trifluoromethyl)-1,2,3,4- tetrahydroquinolin-4-yl)amino)pyrimidin-5-yl)oxy)butanoic acid; 1(2H)-Quinolinecarboxylic acid, 4-(((3,5-bis(trifluoromethyl)phenyl)methyl)(5-(3-carboxypropoxy)-2-pyrimidinyl)amino)-2-ethyl-3,4-dihydro-6-(trifluoromethyl)-,
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 722.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 12 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 17 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cardiovascular disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BE2Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
