Details of the Drug
General Information of Drug (ID: DMBKX3C)
| Drug Name |
VESNARINONE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
vesnarinone; 81840-15-5; Arkin-Z; Piteranometozine; OPC-8212; Arkin; Vesnarinonum [Latin]; Vesnarinona [Spanish]; UNII-5COW40EV8M; OPC 8212; Vesnarinone [USAN:INN:JAN]; CCRIS 1451; DRG-0210; BRN 5644229; 5COW40EV8M; CHEMBL17423; 6-(4-(3,4-Dimethoxybenzoyl)-1-piperazinyl)-3,4-dihydro-2(1H)-; 1-(1,2,3,4-Tetrahydro-2-oxo-6-quinolyl)-4-veratroylpiperazine; 6-[4-(3,4-dimethoxybenzoyl)piperazin-1-yl]-3,4-dihydro-1H-quinolin-2-one; 3,4-Dihydro-6-(4-(3,4-dimethoxybenzoyl)-1-piperazinyl)-2(1H)-quinolinone
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
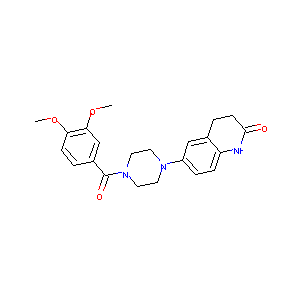 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 395.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
