Details of the Drug
General Information of Drug (ID: DMBSFFK)
| Drug Name |
Giredestrant
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Giredestrant; 1953133-47-5; GDC-9545; RO7197597; Giredestrant [INN]; Giredestrant [USAN]; RO-7197597; 28P3DU6DB3; GDC9545; 3-[(1R,3R)-1-[2,6-difluoro-4-[[1-(3-fluoropropyl)azetidin-3-yl]amino]phenyl]-3-methyl-1,3,4,9-tetrahydropyrido[3,4-b]indol-2-yl]-2,2-difluoropropan-1-ol; 3-((1R,3R)-1-(2,6-Difluoro-4-((1-(3-fluoropropyl)azetidin-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido(3,4-b)indol-2-yl)-2,2-difluoropropan-1-ol; 3-((1R,3R)-1-(2,6-Difluoro-4-((1-(3-fluoropropyl)azetidin-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)-2,2-difluoropropan-1-ol; 3-[(1R,3R)-1-(2,6-difluoro-4-{[1-(3-fluoropropyl)azetidin-3-yl]amino}phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl]-2,2-difluoropropan-1-ol; Giredestrant [USAN:INN]; UNII-28P3DU6DB3; GIREDESTRANT [WHO-DD]; CHEMBL4650316; SCHEMBL17839430; GTPL12715; EX-A3541; BDBM50572808; NSC827275; RG6171; WHO 11226; AKOS040733254; AT22918; NSC-827275; RG-6171; compound 35 [PMID: 34251202]; AC-37111; MS-29682; HY-109176; CS-0116370; (1R,3R)-1-(2,6-DIFLUORO-4-((1-(3-FLUOROPROPYL)-3-AZETIDINYL)AMINO)PHENYL)-.BETA.,.BETA.-DIFLUORO-1,3,4,9-TETRAHYDRO-3-METHYL-2H-PYRIDO(3,4-B)INDOLE-2-PROPANOL; 2H-PYRIDO(3,4-B)INDOLE-2-PROPANOL, 1-(2,6-DIFLUORO-4-((1-(3-FLUOROPROPYL)-3-AZETIDINYL)AMINO)PHENYL)-.BETA.,.BETA.-DIFLUORO-1,3,4,9-TETRAHYDRO-3-METHYL-, (1R,3R)-; 2H-Pyrido(3,4-b)indole-2-propanol, 1-(2,6-difluoro-4-((1-(3-fluoropropyl)-3-azetidinyl)amino)phenyl)-beta,beta-difluoro-1,3,4,9-tetrahydro-3-methyl-, (1R,3R)-; 3-((1R,3R)-1-(2,6-difluoro-4-((1-(3-fluoropropyl)azetidin-3-yl)amino)phenyl)-3-methyl-3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)-2,2-difluoropropan-1-ol; 3-[(1R,3R)-1-[2,6-difluoro-4-[[1-(3-fluoropropyl)azetidin-3-yl]amino]phenyl]-3-methyl-1,3,4,9-tetrahydropyrido[3,4-b]indol-2-yl]-2,2-difluoro-propan-1-ol; ZNM
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
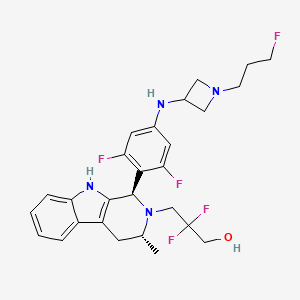 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
