Details of the Drug
General Information of Drug (ID: DMBTDZL)
| Drug Name |
3-Phenyl-pyrrolidine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3-Phenylpyrrolidine; 936-44-7; 3-Phenyl-pyrrolidine; Pyrrolidine, 3-phenyl-; (Pyrrolidin-3-yl)benzene; CHEMBL75207; PRRFFTYUBPGHLE-UHFFFAOYSA-N; F2189-0088; 3-phenyl-pyrolidine; 3-phenyl-pyrollidine; 4-phenyl-pyrrolidine; PubChem22374; BAS 03334576; AC1MK0OA; SCHEMBL4122; AC1Q1H4Y; (+/-)-3-phenyl-pyrrolidine; 3-phenylpyrrolidine, AldrichCPR; KS-00001OYR; CTK3I6515; DTXSID60389848; MolPort-000-006-168; HMS1704P05; 3AAX-0-0; BBL008865; ANW-54854; SBB010168; KM3208; BDBM50144660; STK006609; AKOS000674061; AKOS016051772; MCULE-5600189216
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
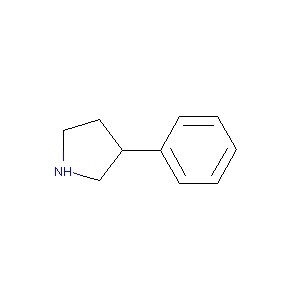 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 147.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
