Details of the Drug
General Information of Drug (ID: DMBZJT5)
| Drug Name |
(3-((1H-imidazol-1-yl)methyl)phenyl)methanol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
[3-(1H-Imidazol-1-ylmethyl)phenyl]methanol; 151055-79-7; CHEMBL597368; W-205714; [3-(imidazol-1-ylmethyl)phenyl]methanol; Benzenemethanol, 3-(1H-imidazol-1-ylmethyl)-; (3-((1H-imidazol-1-yl)methyl)phenyl)methanol; SCHEMBL3092795; DTXSID60439216; MolPort-000-143-255; ZX-AT016572; BDBM50307217; ZINC12370274; SBB090815; FCH921386; AKOS006343964; RP03595; [3-(imidazolylmethyl)phenyl]methan-1-ol; DB-063877; FT-0703495; Y7599; Benzenemethanol,3-(1H-imidazol-1-ylmethyl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
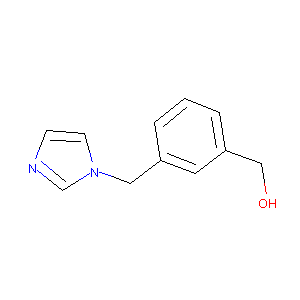 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 188.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
