Details of the Drug
General Information of Drug (ID: DMC7DYX)
| Drug Name |
Edoxudine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5-ethyl-1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-dihydropyrimidine-2,4-d ione; ORF-15817; RWJ-15817; Edurid (Salt/Mix); 1-(2-deoxypentofuranosyl)-5-ethylpyrimidine-2,4(1h,3h)-dione; AC1L1CAR; .beta.-5-Ethyldeoxyuridine; TimTec1_004024; SCHEMBL65580; MLS001360450; AC1Q69H9; 5-ethyl-1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione; .beta.-5-Ethyl-2'-deoxyuridine; XACKNLSZYYIACO-UHFFFAOYSA-N; HMS3056J10; HMS3369L22; HMS1545G20; EDU; AKOS024282522; 5-Ethyl-2'-deoxyuridine ; Edoxudine; MCULE-3445830855; ST056929
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Herpes simplex virus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
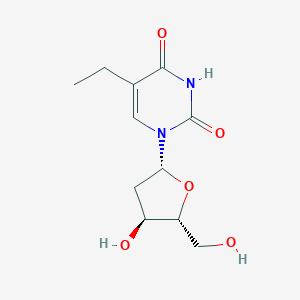 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 256.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
