Details of the Drug
General Information of Drug (ID: DMCERAB)
| Drug Name |
Sudapyridine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Sudapyridine; WX-081; 1859978-72-5; 7X86XPE5TG; GTPL13002; GLXC-25909; HY-146158; CS-0527337; (1R,2S)-1-[5-(4-chlorophenyl)-2-methoxypyridin-3-yl]-4-(dimethylamino)-2-naphthalen-1-yl-1-phenylbutan-2-ol; (alphaS,betaR)-5-(4-Chlorophenyl)-alpha-[2-(dimethylamino)ethyl]-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-3-pyridineethanol; 3-Pyridineethanol, 5-(4-chlorophenyl)-alpha-[2-(dimethylamino)ethyl]-2-methoxy-alpha-1-naphthalenyl-beta-phenyl-, (alphaS,betaR)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
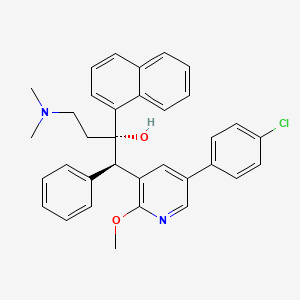 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
