Details of the Drug
General Information of Drug (ID: DMCOMLS)
| Drug Name |
APY29
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1216665-49-4; APY 29; APY-29; N~2~-1h-Benzimidazol-5-Yl-N~4~-(3-Cyclopropyl-1h-Pyrazol-5-Yl)pyrimidine-2,4-Diamine; N2-1H-Benzimidazol-6-yl-N4-(5-cyclopropyl-1H-pyrazol-3-yl)-2,4-pyrimidinediamine; N2-(1H-benzo[d]imidazol-6-yl)-N4-(5-cyclopropyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine; (E)-N-(6-((5-cyclopropyl-1H-pyrazol-3-yl)imino)-1,6-dihydropyrimidin-2-yl)-1H-benzo[d]imidazol-6-amine; APY 29;APY-29; CHEMBL1231018; SCHEMBL12045464; SCHEMBL20438003; SCHEMBL21067744; EX-A305; BCP09071; MFCD28023582; s6623; ZINC40163635; AKOS024458384; DB07382; 2-N-(3H-benzimidazol-5-yl)-4-N-(5-cyclopropyl-1H-pyrazol-3-yl)pyrimidine-2,4-diamine; NCGC00379217-02; NCGC00379217-03; AK547105; BS-14553; DA-47145; HY-17537; QC-11386; AB0098417; FT-0700119; J3.589.967G; Q27096602; 2,4-Pyrimidinediamine, N2-1H-benzimidazol-6-yl-N4-(5-cyclopropyl-1H-pyrazol-3-yl)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
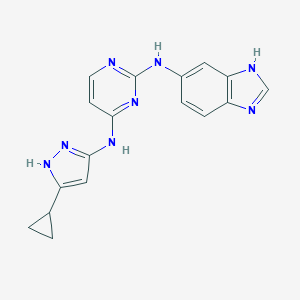 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 332.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
