Details of the Drug
General Information of Drug (ID: DMCXR4M)
| Drug Name |
2',3'-Dideoxycytidine-5'-Monophosphate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
104086-76-2; ((2S,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)tetrahydrofuran-2-yl)methyl dihydrogen phosphate; 5'-Cytidylic acid, 2',3'-dideoxy-; ddCMP; 2',3'-DIDEOXYCYTIDINE-5'-MONOPHOSPHATE; BRN 0556994; 2',3'-Dideoxycytidine 5'-monophosphate; CHEMBL1232305; C9H14N3O6P; [(2S,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)tetrahydrofuran-2-yl]methyl dihydrogen phosphate; AC1L9JZG; SCHEMBL454264; dideoxycytidine 5'-monophosphate; ZINC1611080; 0954AA; BDBM50349537; AKOS016009455; DB02883; LS-59086; AJ-28234; AK112219; KB-204945; AX8123167
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
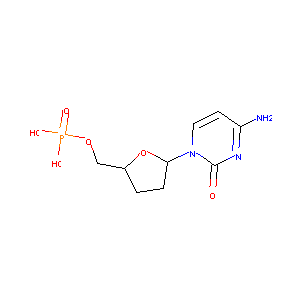 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 291.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
