Details of the Drug
General Information of Drug (ID: DMDIYFH)
| Drug Name |
PRE-084
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pre-084; 138847-85-5; 2-Morpholinoethyl 1-Phenylcyclohexanecarboxylate; 2-(4-Morpholino)ethyl-1-phenylcyclohexane-1-carboxylate; CHEMBL305881; 2-morpholin-4-ylethyl 1-phenylcyclohexane-1-carboxylate; Cyclohexanecarboxylic acid, 1-phenyl-, 2-(4-morpholinyl)ethyl ester; Tocris-0589; Lopac-P-2607; AC1L2Q9Z; Lopac0_000927; PRE084; GTPL6678; SCHEMBL5564153; CHEBI:93007; DTXSID40160819; EX-A911; BCP25418; BDBM50039197; ZINC25756875; AKOS027439951; CCG-205008; NCGC00024669-03; NCGC00024669-02; NCGC00024669-01; NCGC00015808-04
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
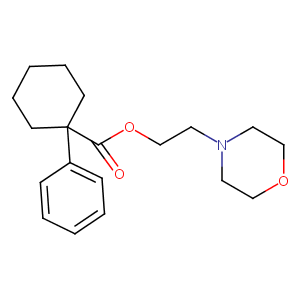 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 317.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Aging skin | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EE40 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
