Details of the Drug
General Information of Drug (ID: DMDQS4W)
| Drug Name |
ASC-J9
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dimethylcurcumin; 52328-98-0; 917813-54-8; ASCJ-9; (1E,4Z,6E)-1,7-bis(3,4-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one; 1,7-Bis-(3,4-dimethoxyphenyl)-5-hydroxy-hepta-1,4,6-trien-3-one; 1,7-Bis-(3,4-dimethoxy-phenyl)-5-hydroxy-hepta-1,4,6-trien-3-one; GO-Y-025; (1E,4E,6E)-1,7-Bis(3,4-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one; Dimethylcurcumin (ASC-J9); 1,7-Bis(3,4-dimethoxyphenyl)-5-hydroxyhepta-1,4,6-trien-3-one; SCHEMBL3487103; CHC-004; DTXSID10200352; EX-A927; AMY15692; 3454AH; MFCD12912341; MFCD22123809; NSC734923; RSC004738; s6630; AKOS015891371; AKOS025311328; ZINC100007120; CS-0533; DB06133; LS40141; NSC-734923; SB18767; 1,4,6-Heptatrien-3-one, 1,7-bis(3,4-dimethoxyphenyl)-5-hydroxy-, (1E,4Z,6E)--; 1,4,6-Heptatrien-3-one, 1,7-bis(3,4-dimethoxyphenyl)-5-hydroxy-, (Z,E,E)-; AC-31058; DS-14720; HY-15194; ASC-J9,CAS:52328-98-0; W9512; J3.606.945G; A11300; W-5544; GO-Y025; Dimethylcurcumin; ASC J9; GO Y025; 1, 4-dimethoxyphenyl)-1, 6-heptadiene-3,5-dione; (1E,6E)-1,7-Bis(3,4-dimethoxyphenyl)-5-hydroxy-1,4,6-heptatriene-3-one; 1,7-Bis-(3,4-dimethoxy-phenyl)- 5-hydroxy-hepta-1,4,6-trien-3-one; (1E,4Z,6E)-1,7-bis(3,4-dimethoxyphenyl)-5-hydroxy-1,4,6-heptatrien-3-one; (1E,4Z,6E)-1,7-Bis(3,4-dimethoxyphenyl)-5-hydroxy-1,4,6-heptatriene-3-one; (1E,4Z,6E)-1,7-bis(3,4-dimethoxyphenyl)-5-hydroxy-hepta-1,4,6-trien-3-one; 115851-85-9
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
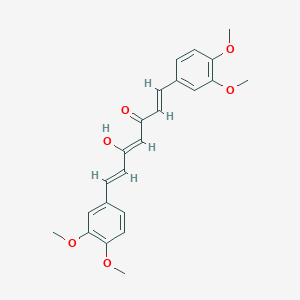 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 396.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | End-stage renal disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GB61.5 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
