Details of the Drug
General Information of Drug (ID: DMDU6GN)
| Drug Name |
JNJ-479655
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1428327-31-4; N-((4-(4-phenylpiperazin-1-yl)tetrahydro-2H-pyran-4-yl)methyl)-2-(phenylthio)nicotinamide; JNJ-47965567; JNJ 47965567; N-{[4-(4-Phenylpiperazin-1-Yl)oxan-4-Yl]methyl}-2-(Phenylsulfanyl)pyridine-3-Carboxamide; P2X Antagonist III; antagonist JNJ47965567; GTPL7538; CHEMBL2338352; MolPort-035-941-198; ZINC95590396; AKOS025142079; JNJ47965567; NCGC00387264-01; J-115; JNJ-47965567, > Z2235332565; N-[[4-(4-phenylpiperazin-1-yl)oxan-4-yl]methyl]-2-phenylsulfanylpyridine-3-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
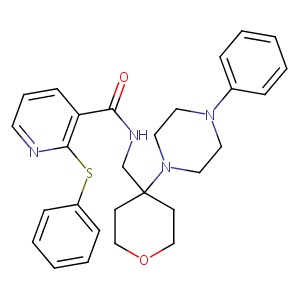 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 488.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Alzheimer disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
