Details of the Drug
General Information of Drug (ID: DMDUB63)
| Drug Name |
7-amino-4-hydroxy-2-naphthalenesulfonic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
87-02-5; J acid; 7-AMINO-4-HYDROXY-2-NAPHTHALENESULFONIC ACID; 7-amino-4-hydroxynaphthalene-2-sulfonic acid; Isogamma acid; I acid; 2-Naphthalenesulfonic acid, 7-amino-4-hydroxy-; Kyselina I; 2-Amino-5-naphthol-7-sulfonic Acid; Kyselina I [Czech]; Aminonaphthol sulfonic acid J; 6-Amino-1-naphthol-3-sulfonic acid; CCRIS 8989; EINECS 201-718-9; NSC 31510; Kyselina 2-amino-5-naftol-7-sulfonova; BRN 2217192; UNII-9A1IU1C93L; MLS002638012; CHEMBL220259; 9A1IU1C93L; CHEBI:87316; 7-Amino-4-hydroxynaphthalene-2-sulphonic acid; Kyselina 6
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
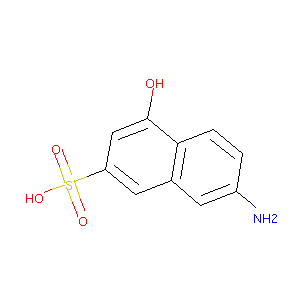 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 239.25 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
