Details of the Drug
General Information of Drug (ID: DME50RZ)
| Drug Name |
2-(benzyloxy)naphthalene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Benzyl 2-naphthyl ether; 613-62-7; 2-(benzyloxy)naphthalene; 2-Benzyloxynaphthalene; Naphthalene, 2-(phenylmethoxy)-; 2-(phenylmethoxy)naphthalene; 2-Benzyloxy-naphthalene; 2-phenylmethoxynaphthalene; CHEMBL146439; Q-200695; 2-(Phenylmethoxy)-naphthalene; Benzyl2-naphthylether; 2-Phenylmethoxy naphthalenen; b-naphthylbenzyl ether; ss-Naphtholbenzylaether; benzyl-2-naphthylether; ACMC-209mru; AI3-00945; AC1L3VTE; beta-naphthyl benzyl ether; EC 405-490-3; SCHEMBL51460; KSC493E6R; CTK3J3268; DTXSID80210201; MolPort-002-476-516
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
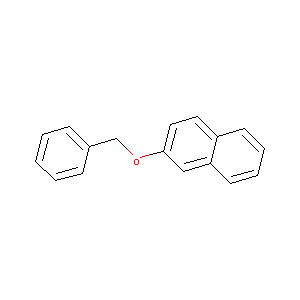 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 234.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
