Details of the Drug
General Information of Drug (ID: DMEAOYJ)
| Drug Name |
Ponesimod
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
854107-55-4; UNII-5G7AKV2MKP; 5G7AKV2MKP; CHEMBL1096146; Ponesimod [USAN:INN]; Ponesimod,ACT-128800; Ponesimod (ACT-128800); GTPL9320; SCHEMBL15477937; SCHEMBL15477934; DTXSID50234631; MolPort-035-681-391; MolPort-046-033-541; EX-A1417; ZINC34509627; s8241; BDBM50316768; AKOS022180266; DB12016; Compound 8bo [PMID:20446681]; HY-10569; KB-72962; AS-35140; AJ-89002; (2Z,5Z)-5-(3-Chloro-4-((2R)-2,3-dihydroxypropoxy)phenylmethylidene)-3-(2-methylphenyl)-2-(propylimino)-1,3-thiazolidin-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
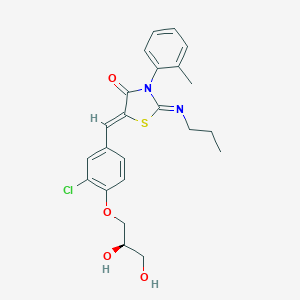 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 461 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
