Details of the Drug
General Information of Drug (ID: DMEXZQ5)
| Drug Name |
2-Phenyl-3-piperidin-4-yl-1H-indole
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
221109-26-8; 2-PHENYL-3-(PIPERIDIN-4-YL)-1H-INDOLE; 2-Phenyl-3-(4-piperidinyl)-1H-indole; 2-Phenyl-3-piperidin-4-yl-1H-indole; CHEMBL295559; SCHEMBL7962369; CTK4E8621; DTXSID60432328; FWPAVENSJPMGJO-UHFFFAOYSA-N; ZINC2580888; CP-220; ANW-46494; BDBM50095050; AKOS015898489; AJ-42917; 3-(Piperidin-4-yl)-2-phenyl-1H-indole; 1H-Indole,2-phenyl-3-(4-piperidinyl)-; TC-135239; ST2404535; KB-231980; AX8078102; W4568; FT-0707553; AM20030371; P13451
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
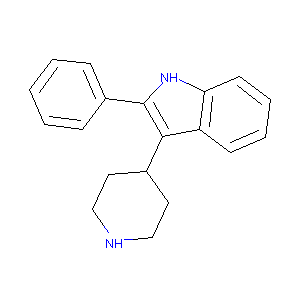 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 276.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
