Details of the Drug
General Information of Drug (ID: DMEZGWP)
| Drug Name |
Zabedosertib
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Zabedosertib; 1931994-81-8; N-[6-(1-hydroxy-1-methyl-ethyl)-2-(2-methylsulfonylethyl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; Zabedosertib [INN]; BAY-1834845; N1GRK350ZM; BAY1834845; BAY 1834845; 2-Pyridinecarboxamide, N-(6-(1-hydroxy-1-methylethyl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)-; N-(6-(1-Hydroxy-1-methylethyl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)-2-pyridinecarboxamide; N-(6-(2-Hydroxypropan-2-yl)-2-(2-(methanesulfonyl)ethyl)- 2H-indazol-5-yl)-6-(trifluoromethyl)pyridine-2- carboxamide; N-(6-(2-Hydroxypropan-2-yl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)picolinamide; N-[6-(2-hydroxypropan-2-yl)-2-(2-methylsulfonylethyl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; UNII-N1GRK350ZM; SCHEMBL17785221; GTPL11415; OQAMEEFUUFJZRS-UHFFFAOYSA-N; BDBM395297; EX-A5143; US10308634, Example 12; MFCD32900903; AKOS040755617; SB74225; MS-28697; SY323200; HY-139374; CS-0198831; D96922; N-[6-(2-Hydroxy-2-propyl)-2-[2-(methylsulfonyl)ethyl]-2H-indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; N-{6-(2-Hydroxypropan-2-yl)-2-[2-(methylsulphonyl)ethyl]-2H-indazol-5-yl}-6-(trifluoromethyl)pyridine-2-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
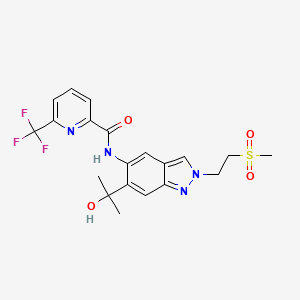 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
