Details of the Drug
General Information of Drug (ID: DMF0HWX)
| Drug Name |
Hypoestoxide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
88498-46-8; hypoestoxide; (1R,3S,5S,8S,10S,12R,13S)-5,10,17,17-Tetramethyl-14-methylene-15-oxo-4,9-dioxatetracyclo[11.3.1.03,5.08,10]heptadecan-12-yl acetate; Hypoestoxide,hypoestes rosea; PD132594; [(1R,3S,5S,8S,10S,12R,13S)-5,10,17,17-tetramethyl-14-methylidene-15-oxo-4,9-dioxatetracyclo[11.3.1.03,5.08,10]heptadecan-12-yl] acetate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
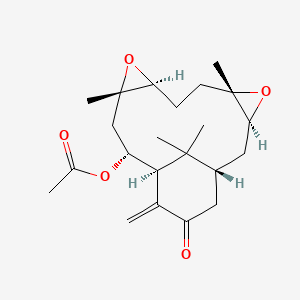 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
