Details of the Drug
General Information of Drug (ID: DMG0C81)
| Drug Name |
Deferoxamine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
deferoxamine; Desferrioxamine B; DESFERRIOXAMINE; Deferoxamine B; Deferrioxamine B; 70-51-9; Deferrioxamine; Deferoxamin; Deferoxaminum; DFOM; Desferin; Desferral; Desferrin; Desferex; Desferan; N-Benzoylferrioxamine B; Desferal; DF B; DFOA; Deferoxamide B; Deferoxamina; NSC-527604; Ferrioxamine B, N-benzoyl-; Desferriferrioxamin B; UNII-J06Y7MXW4D; Deferoxamine [USAN:INN]; Deferoxaminum [INN-Latin]; Deferoxamina ; Deferoxamine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
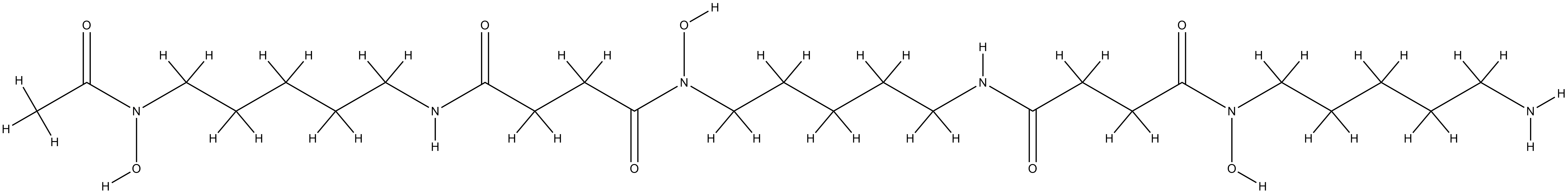 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
