Details of the Drug
General Information of Drug (ID: DMG0PHU)
| Drug Name |
CYNAROPICRIN
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cynaropicrin; aguerin; 35730-78-0; CHEMBL374146; CHEBI:4038; UNII-M9233789I9; M9233789I9; [(3aR,4S,6aR,8S,9aR,9bR)-8-hydroxy-3,6,9-trimethylidene-2-oxo-3a,4,5,6a,7,8,9a,9b-octahydroazuleno[4,5-b]furan-4-yl] 2-(hydroxymethyl)prop-2-enoate; AC1Q69JD; AC1L3O6W; SCHEMBL1711811; MEGxp0_001095; ACon1_000045; MolPort-001-741-258; ZINC4098049; HY-N2350; BDBM50194430; AKOS032971358; MCULE-5051144608; CS-8041; NCGC00168845-01; NCGC00168845-02; 2-Propenoic acid, 2-(hydroxymethyl)-, (3aR,4S,6aR,8S,9aR,9bR)-dodecahydro-8-hydroxy-3,6,9-tris(m
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
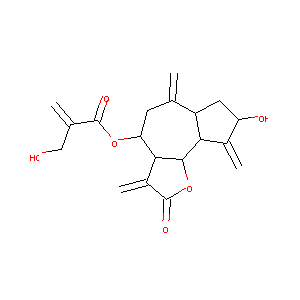 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 346.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
