Details of the Drug
General Information of Drug (ID: DMG7SQK)
| Drug Name |
Rescinnamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Anapral; Anaprel; Apolon; Apoterin; Cartric; Cinamine; Cinatabs; Cinnaloid; Cinnasil; Moderil; Normorescina; Paresinan; Raupyrol; Raurescin; Raurescine; Recinnamine; Recitensina; Rescaloid; Rescamin; Rescidan; Rescin; Rescinamina; Rescinnamin; Rescinnamina; Rescinnaminum; Rescinpal; Rescisan; Rescitens; Resealoid; Reserpinene; Reserpinin; Reserpinine; Resipal; Reskinnamin; Rozex; Scinnamina; Tenamine; Tuareg; Apoterin S; Methyl trimethoxycinnamoylreserpate; Rescinnamina [DCIT]; Trimethoxy cinnamoyl reserpate de methyl; Trimethoxy cinnamoyl reserpate de methyl [French]; Trimethoxycinnamoyl methyl reserpate; Tsuruselpi S; Anaprel (TN); Cinnasil (TN); Moderil (TN); NP-011016; Rescinamina [INN-Spanish]; Rescinnamine (VAN); Rescinnaminum [INN-Latin]; Resepinine (C35 alkaloid); Reserpinine (C35 alkaloid); Reserpinine (VAN); Tsuruselpi S (TN); Rescinnamine (JAN/INN); Rescinnamine [BAN:INN:JAN]; Methyl reserpate 3,4,5-trimethoxycinnamic acid ester; Reserpic acid methyl ester 3,4,5-trimethoxycinnamate; Methyl 18-O-(3,4,5-trimethoxycinnamoyl)reserpate; O-(3,4,5-Trimethoxy-trans-cinnamoyl) methyl reserpate; 3,4,5-Trimethoxycinnamic acid, methyl reserpate; 3,4,5-Trimethoxycinnamoyl methyl reserpate; 3,4,5-Trimethylcinnamic acid, ester with methyl reserpate; 3,4,5-Trimethylcinnamoyl methyl reserpate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
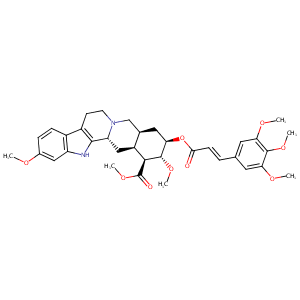 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 634.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hypertension | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00-BA04 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
