Details of the Drug
General Information of Drug (ID: DMGLZ26)
| Drug Name |
Practolol
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dalzic; Eraldin; Practololo; Practololum; Praktololu; Teranol; Eralzdin Practolol; Practololo [DCIT]; Praktololu [Polish]; AY 21011; Cardiol (TN); Cordialina (TN); Dalzic (TN); Eraldin (TN); Eraldina (TN); Practololum [INN-Latin]; Praktol (TN); Pralon (TN); Teranol (TN); Practolol [USAN:BAN:INN]; N-{4-[2-hydroxy-3-(isopropylamino)propoxy]phenyl}acetamide; N-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide; N-[4-({2-hydroxy-3-[(1-methylethyl)amino]propyl}oxy)phenyl]acetamide; N-{4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl}acetamide; N-(4-(2-Hydroxy-3-((1-methylethyl)amino)propoxy)phenyl)acetamide; (+-)-Practolol; 1-(4-Acetamidophenoxy)-3-isopropylamino-2-propanol; 4'-(2-Hydroxy-3-(isopropylamino)propoxy)acetanilide
|
|||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||
| Therapeutic Class |
Antiarrhythmic Agents
|
|||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||
| Structure |
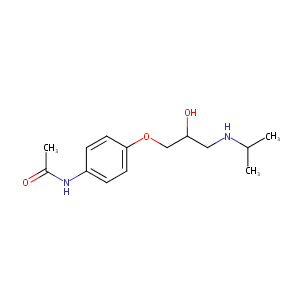 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 266.34 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.8 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
