Details of the Drug
General Information of Drug (ID: DMGQC2F)
| Drug Name |
2-(2-(pyrrolidin-1-yl)ethyl)pyridine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-(2-Pyrrolidinoethyl)Pyridine; 6311-90-6; CHEMBL270595; 2-[2-(Pyrrolidin-1-yl)ethyl]pyridine; N-(2-(2-pyridyl)ethyl)pyrrolidine; NSC42644; 2-[2-(1-Pyrrolidinyl)ethyl]pyridine; AC1Q4VNT; AC1L60PK; AC1Q28PR; SCHEMBL868571; 2-(2-Pyrrolizinoethyl)pyridine; CTK5B7636; DTXSID60285694; OXEFHEYEQUISKC-UHFFFAOYSA-N; ZINC393798; NSC-42644; 2-(2-pyrrolidin-1-ylethyl)pyridine; BDBM50372289; AKOS006243249; MCULE-8128571557; 2-(2-Pyrrolidin-1-yl-ethyl)-pyridine; Pyridine,2-[2-(1-pyrrolidinyl)ethyl]-; 2-[2-(1-Pyrrolidinyl)ethyl]pyridine #
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
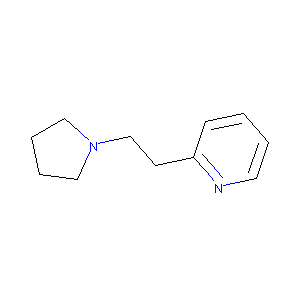 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 176.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
