Details of the Drug
General Information of Drug (ID: DMGXD5T)
| Drug Name |
Brotizolam
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Brotizolamum; Brotizolamum [INN-Latin]; Lendorm; Lendormin; Mederantil; Sintonal; UMSGKTJDUHERQW-UHFFFAOYSA-N; WE 941; WE-941; brotizolam; 2-Bromo-4-(2-chlorophenyl)-9-methyl-6H-thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine; 2-Bromo-4-(o-chlorophenyl)-9-methyl-6H-thieno(3,2-f)-s-triazolo(4,3-a)(1,4)diazepine; 2-bromo-4-(2-chlorophenyl)-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine; 57801-81-7; 5XZM1R3DKF; BRN 0839277; C15H10BrClN4S; CHEMBL32479; EINECS 260-964-5; NCGC00183872-01; UNII-5XZM1R3DKF
|
|||||
| ATC Code | ||||||
| Structure |
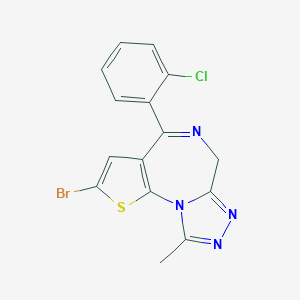 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 393.7 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | |||||
| Rotatable Bond Count (rotbonds) | 1 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
