Details of the Drug
General Information of Drug (ID: DMH1DYU)
| Drug Name |
MSC1936369B
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pimasertib; 1236699-92-5; AS703026; AS-703026; Pimasertib (AS-703026); MSC1936369B; UNII-6ON9RK82AL; AS 703026; (S)-N-(2,3-dihydroxypropyl)-3-(2-fluoro-4-iodophenylamino)isonicotinamide; 6ON9RK82AL; N-[(2S)-2,3-Dihydroxypropyl]-3-[(2-fluoro-4-iodophenyl)amino]-4-pyridinecarboxamide; 1204531-26-9; C15H15FIN3O3; N-[(2S)-2,3-dihydroxypropyl]-3-(2-fluoro-4-iodo-anilino)pyridine-4-carboxamide; N-((2S)-2,3-dihydroxypropyl)-3-((2-fluoro-4-iodophenyl)amino)pyridine-4-carboxamide; Pimasertib [USAN:INN]
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
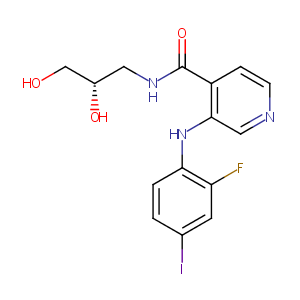 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 431.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
