| Drug Name |
Cloranolol
|
| Synonyms |
Chloranolol-d9; Cloranolol; Cloranolol (INN); Cloranolol [INN]; Cloranolol-d9; Cloranololum; Cloranololum [INN-Latin]; Tobanum-d9; chloranolol; chlorpropanol; 1-(2,5-dichlorophenoxy)-3-tertiary-butylamino-2-propanol; 1-(tert-Butylamino)-3-(2,5-dichlorophenoxy)-2-propanol; 1-(tert-butylamino)-3-(2,5-dichlorophenoxy)propan-2-ol; 2-Propanol, 1-(2,5-dichlorophenoxy)-3-((1,1-dimethylethyl)amino)-; 39563-28-5; AC1L240Q; C13H19Cl2NO2; CHEBI:135217; CHEMBL156791; Gyki 41099; SCHEMBL80493; tobanum, hydrochloride, (+-)-isomer
|
| ATC Code |
- C07AA27: Cloranolol
- C07AA: Beta blocking agents, non-selective
- C07A: BETA BLOCKING AGENTS
- C07: BETA BLOCKING AGENTS
- C: CARDIOVASCULAR SYSTEM
|
| Structure |
|
 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
292.2 |
|
| Logarithm of the Partition Coefficient (xlogp) |
3.1 |
| Rotatable Bond Count (rotbonds) |
6 |
| Hydrogen Bond Donor Count (hbonddonor) |
2 |
| Hydrogen Bond Acceptor Count (hbondacc) |
3 |
| Chemical Identifiers |
- Formula
- C13H19Cl2NO2
- IUPAC Name
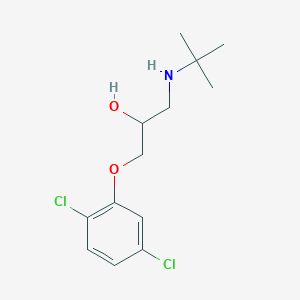
1-(tert-butylamino)-3-(2,5-dichlorophenoxy)propan-2-ol - Canonical SMILES
-
CC(C)(C)NCC(COC1=C(C=CC(=C1)Cl)Cl)O
- InChI
-
XYCMOTOFHFTUIU-UHFFFAOYSA-N
- InChIKey
-
1S/C13H19Cl2NO2/c1-13(2,3)16-7-10(17)8-18-12-6-9(14)4-5-11(12)15/h4-6,10,16-17H,7-8H2,1-3H3
|
| Cross-matching ID |
- PubChem CID
- 65814
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR0357
|
|
|
|
|
|
|
|

